Newsroom
Newsroom
News and Updates about Intera Oncology


April 12, 2023 - Intera Oncology Showcases Intera 3000 Hepatic Artery Infusion (HAI) Pump at the Annual Cholangiocarcinoma Foundation Conference
Intera Oncology® Inc., manufacturer of the only FDA-approved pump for Hepatic Artery Infusion (HAI) therapy for the treatment of intrahepatic cholangiocarcinoma (bile duct cancer confined to the liver) and colorectal cancer liver metastases, will showcase its Intera 3000 HAI pump as a sponsor of the 10th annual Cholangiocarcinoma Foundation Conference April 12 to 15 in Salt Lake City, Utah.
January 19, 2023, Boston, MA - Intera 3000, the only FDA-approved implantable pump for HAI therapy, showcased at ASCO Gastrointestinal Cancer Symposium 2023
Intera Oncology® Inc., the manufacturer of the only FDA-approved implantable pump used for hepatic artery infusion (HAI) therapy, will showcase its Intera 3000 HAI Pump at the 2023 ASCO Gastrointestinal Cancer Symposium, taking place at the Moscone West in San Francisco, Calif. from January 19 to 20, 2023.
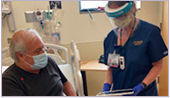
Summer 2022, Davis, CA - First in region targeted liver cancer therapy gives patients new hope

June 15, 2022, Portland, OR - OHSU HAI clinical trial shows promise
November 12, 2021, Birmingham, AL - New program brings more treatment options to patients with colorectal cancer
August 18, 2021, Durham, NC - DukeHealth implants first Intera 3000 Hepatic Arterial Infusion Pump
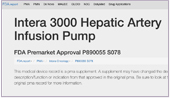
June 2021, Boston - FDA approves manufacture of Intera 3000 Hepatic Arterial Infusion Pump
Intera Oncology is proud to announce that the FDA has approved the manufacture of the Intera 3000 Hepatic Arterial Infusion (HAI) Pump for HAI therapy for patients with colorectal cancer and cholangiocarcinoma. The Intera 3000 is now the only FDA-approved pump for HAI. “We formed Intera Oncology to bring this pump back to market after it was discontinued by another company. We could not let those who stood to benefit from HAI therapy go without that option,” said Michael Gaisford, president and CEO of Intera Oncology. “We are thrilled that we have accomplished our mission and that HAI therapy will be available to those who need it.”

March 2019, Boston - Michael Gaisford joins Intera Oncology President and CEO
Michael Gaisford has been named CEO of Intera Oncology, a medical device company dedicated to ensure hepatic artery infusion therapy is available to any patient who could benefit from it. Gaisford brings 15 years’ experience leading strategy, marketing, and product management teams. Prior to Intera, Gaisford was Director of Marketing for Stratasys Healthcare Solutions where he oversaw global marketing programs across medical and dental markets. At Boston Scientific, he held positions in corporate strategy, marketing and divisional product marketing,. Gaisford previously worked at Genentech, CVS/Pharmacy, and McKinsey & Company. He holds an MBA from the Tuck School of Business at Dartmouth and BS from Stanford University.
February 2019, Boston - Intera Oncology launches to save HAI pump
Two doctors determined to resurrect a medical pump that increases survival of some patients with colorectal cancer and cholangiocarcinoma have launched Intera Oncology. Drs. Jonathan Reis and David Dove both have loved ones who benefitted from the only FDA-approved pump that delivers chemotherapy directly to the liver via “hepatic artery infusion therapy” (HAI). When they found that the pump was to be discontinued, the doctors formed Intera Oncology to once again make the pump available to those who may benefit from it.

April 25, 2018, New York - A lifesaving pump for cancer patients is being phased out
New York Times article discusses abrupt discontinuation of HAI Pump.
24/7 Clinical Call : USA & Canada (800) 660-2660
24/7 Clinical Call : USA & Canada (800) 660-2660