Hepatic Artery Infusion Therapy
Hepatic Artery Infusion Therapy
A powerful therapy for patients living with colorectal cancer liver metastases and intrahepatic cholangiocarcinoma
Colorectal Cancer Liver Metastases (CRLM)
HAI therapy can benefit patients with CRLM with the potential to:
Reduce tumor burden, improve disease control in the liver and increase likelihood of conversion to surgical resection
For unresectable colorectal cancer liver metastases (CRLM) patients, HAI in combination with systemic chemotherapy demonstrated a significant reduction of tumor burden in the liver, enabled previously unresectable CRLM to be successfully removed 1, and increased survival rates independent of resectability 2.
Prevent or delay recurrence post resection
HAI therapy is the only adjuvant therapy for resected CRLM shown to improve 2-year survival in a randomized trial 3. In patients who undergo CRLM resection, HAI therapy may be used to reduce likelihood of recurrence post-resection without increasing systemic side effects.
Increase overall survival
HAI therapy in combination with systemic chemotherapy significantly improved long term survival for patients with unresectable CRLM independent of resectablity 2, as well as those treated in the adjuvant setting 4.
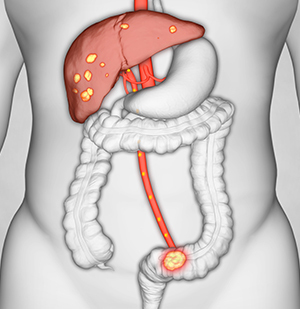
Intrahepatic Cholangiocarcinoma (IHCC)
Hepatic Artery Infusion therapy can benefit patients with IHCC with the potential to:
Increase overall survival
HAI combined with systemic chemotherapy may contribute to substantially extending overall survival for patients with unresectable IHCC. Studies demonstrated that patients treated with HAI achieved 25 months median overall survival and 39.5% survived to 3 years 5, 6.
HAI therapy is increasingly adopted by cancer centers nationwide for patients with CRLM and IHCC
Hepatic Artery Infusion therapy has been used safely for 25 years in thousands of patients*. In recent years, an increasing body of clinical evidence and modernized HAI therapy protocols have led to increased adoption of HAI.
A dedicated community of physicians is actively promoting research and adoption of HAI therapy. Surgical experience, improved techniques and treatment protocols have paved the way for widespread adoption of this therapy. Patients are actively researching therapy options and connect with physicians and medical centers that offer HAI therapy.
Cancer centers nationwide are increasingly adopting HAI therapy as a powerful option for patients with CRLM and IHCC. A growing number of surgical and medical oncology teams are trained and experienced in this therapy today.
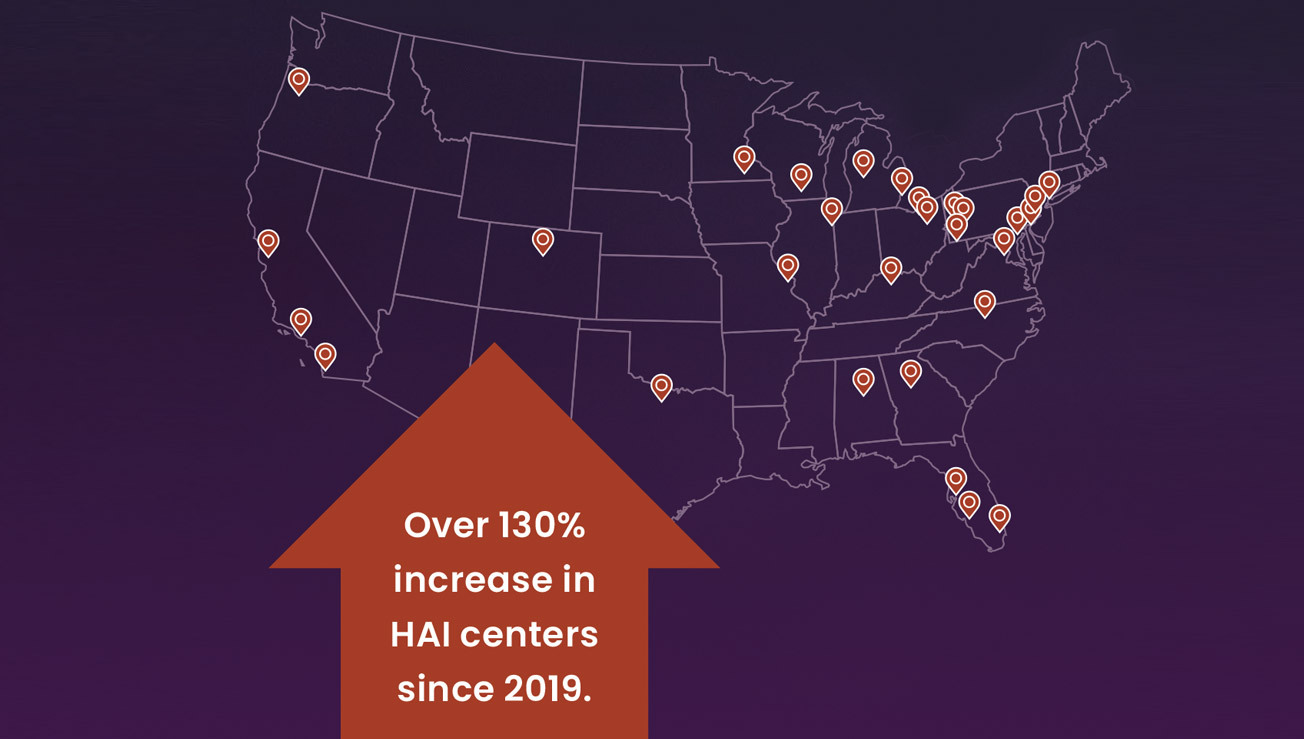
Nine out of the top 10 U.S. Cancer Centers* offer this treatment using the Intera 3000 HAI Pump, the only FDA-approved implantable pump for HAI therapy.
1D’Angelica MI, Correa-Gallego C, Paty PB, et al: Ann Surg; 2015;261:353-360; 2Dhir M, Jones HL, Shuai Y, et al. Annals of Surgical Oncology. 2017;24(1):150-158; 3Kemeny N, et al. New England Journal of Medicine. 1999;341:2039-2048; 4Koerkamp, et. al. J Clin Oncol. 2017 Jun 10; 35(17): 1938–1944; 5Holster J et al., Ann Surg Oncol (2022) 29:5528–5538; 6Cercek A et al. JAMA Oncol Oct 31, 2019
*The Intera 3000 Hepatic Artery Infusion Pump was previously marketed as the Model 3000 Series Pump and Codman® 3000 Series Pump. Codman is a registered trademark of Integra LifeSciences Corporation. Intera Oncology® is not endorsed by, affiliated with, or sponsored by Integra LifeSciences Corporation. *US News and World Report
HAI Therapy
Clinical Evidence
Intera 3000 HAI Pump
24/7 Clinical Call : USA & Canada (800) 660-2660
WS-1008 Rev A
© Copyright 2023 Intera Oncology.
All Rights Reserved. Designed by WollnerStudios.
HAI Therapy
Clinical Evidence
Intera 3000 HAI Pump
24/7 Clinical Call : USA & Canada (800) 660-2660
WS-1008 Rev A